
Marketed by: SV Biotech
Manufactured by: Prime 4 Dia under validation with USFD/ CE / ISO /EU approved product.

Easier: No special equipment needed Intuitive visual interpretation.
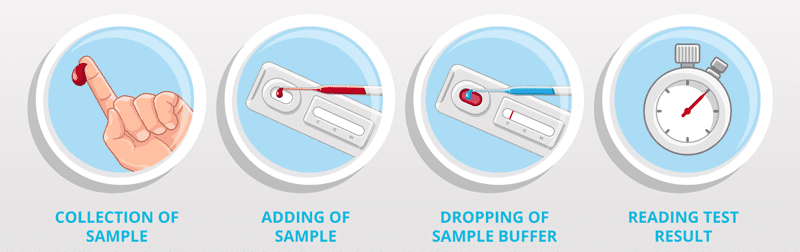
Rapid: Quick sampling by fingertip blood, result in 15 minutes.
Accurate: Qualitative lateral flow Immunoassay for the detection of Corona Virus IgM/IgG nucleoproteins in serum, plasma whole blood specimen.
Application: For suspicious patients with symptoms, mild symptoms, or even without symptoms, also for testing people with close contact of infected patients and people under quarantine control.
It is a rapid chromatographic immunoassay for the qualitative detection of Coronavirus (SARS-CoV-2) IgM and IgG antibody in serum, plasma, whole blood specimens.
P4Detect is USFD/ EU/CE/ISO Certified product manufacturing in South Korea.
It is suited for POC Testing.
Provide information on previous infection.
Detects immune response & provide identification of likely immunity.
Aids in verifying if people can be released from quarantine &/or return to work.
We recommend performing an Internet search in order to find the protocol that best fits your experiments.
Our products are of molecular biology grade. We are confident that our products are of the highest quality and have been handled with the utmost care.
The MSDS are usually listed with the product as well as shipped with your items. If you do not find these documents, please email us at [email protected] and we will make sure to send you copies.
We accept purchase orders from most companies and universities. We also accept NEFT/RTGS/IMPS/LC/Check. If you are a new customer, we ask that you fill out our “New Customer” form to set up your account prior to placing an order. If you have any further questions, you can contact us at [email protected] or +917887884108
Gat. No: 407,408,
A/P- Bhandgaon, Tal- Daund,
Dist- Pune, Maharashtra-412214
02026911273 / 74
[email protected]
![]()
![]()
1. Point of care diagnostics kit
2. Molecular diagnostics kit
3. Food testing kit
4. Clinical lateral flow immunoassay kit